Research
The Zindel Lab is a new and growing research group at ETH Zürich, embedded within the Institute of Molecular Health Sciences. Our focus is on the cellular and molecular biology of tissue repair, with a particular focus on post-surgical repair and scarring in the abdominal cavity. Led by a former abdominal surgeon, the program is deeply translational:
Our goal is to advance knowledge and translate discoveries obtained in basic mechanistic research into tangible clinical benefits for future patients.
To achieve this, we are exploring three main areas of interest:
i) The role of peritoneal cavity macrophages in injury detection and repair.
ii) Mesothelial to mesenchymal transition and migration in repair and scarring.
iii) Foreign body biology in the peritoneal space.
Peritoneal Cavity Macrophages
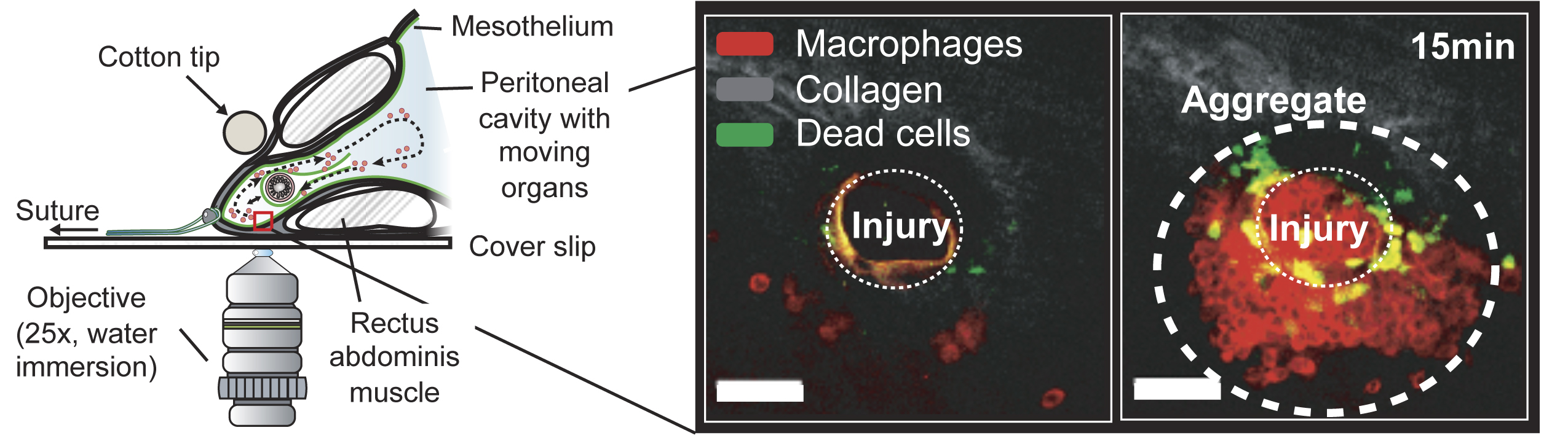
Many vital organs such as the heart, lungs, and the gastrointestinal tract, are contained within body cavities that contain millions of specialized immune cells (macrophages). We and others showed that there are literally millions of these macrophages ready to help. They can detect injuries of the mesothelium, the slippery linings of body cavities within seconds and interfering with the process has significant consequences for successful tissue repair (Figure 1). A core hypothesis of our research is that the activation of cavity macrophages and their intercellular interactions have protective and inflammatory functions in post-surgical injury. Since these cells behave completely differently once they are removed from their niche, we leveraged powerful multi photon microscopes to study them in their native environment through the intact abdominal wall. Together with our clinical collaborators, we run clinical cohort studies validate the translational potential of mechanistic explanatory models that were generated based on observations in the murine system. We ask questions that are of fundamental immunological interest. But the discovery of druggable pathways in this system will be biomedically interesting in surgery, peritoneal cancer, and immunotherapy.
Mesothelial to Mesenchymal Transition
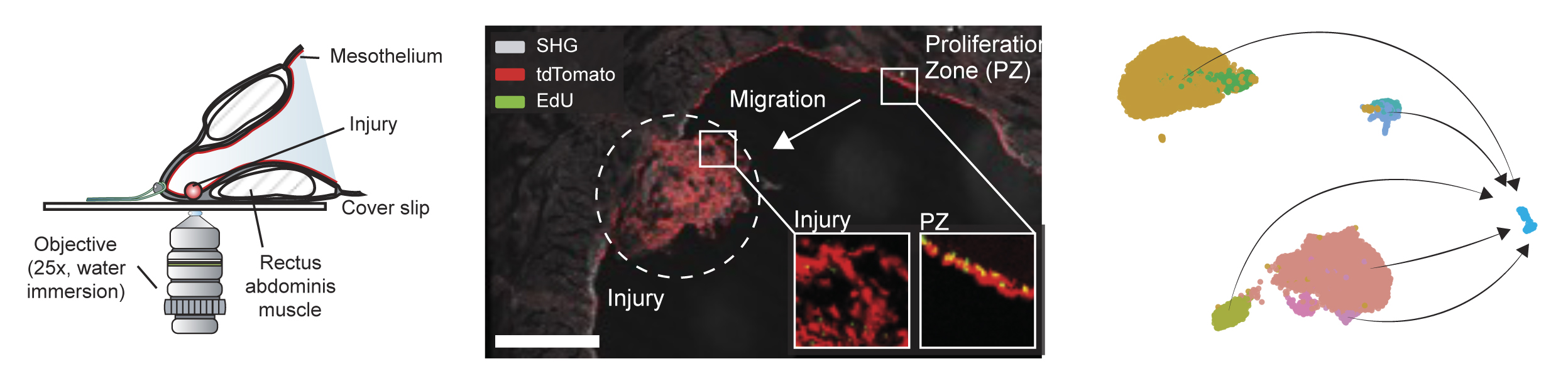
Repair canonically depends on myofibroblasts which can be derived directly from the surrounding healthy mesothelial cells (the slippery lining) through a transformative process called mesothelial to mesenchymal transition or MMT. MMT is very important for the required tissue repair post-surgery, but MMT is also a major driver of pathological post-surgical scarring—also referred to as adhesion formation—that can cause significant health problems down the road (Figure 2). A core hypothesis is therefore, that the cellular reprogramming and migratory behaviour of mesothelial cells is a critical difference between physiological and pathological post-surgical repair. We use transplantation models, intravital microscopy, and human cohort studies, to investigate the biology of MMT. A special focus lies on deciphering the crosstalk between immune cells and mesothelial cells. These questions are biomedically critical to understand repair after abdominal, thoracic, and cardiac surgeries. The challenge to image mesenchymal cells migrating over large distances in living organisms is substantial, but the risk comes with a potential high gain, as this technical deliverable could impact on the field of cellular migration as a whole.
Foreign body biology in the peritoneal space.
Worldwide, millions live with permanent implants in their body cavities such as staples, pacemaker electrodes, or hernia mesh. Indeed, the peritoneal space would be anatomically and physiologically suitable for the implantation of closed-circuit artificial organs. But to date, a major limitation for the intraperitoneal approach is device occlusion by foreign body reaction. A core hypothesis is that evolutionarily conserved serosal immune mechanisms orchestrate a foreign body response, limiting the functionality of implants within serosal body cavities. We collaborate in a transdisciplinary approach with (bio)material engineers (Prof. Dr. Inge Herrmann, Prof. Mark Tibbitt) and ask fundamental immunological as well as directly translational questions.